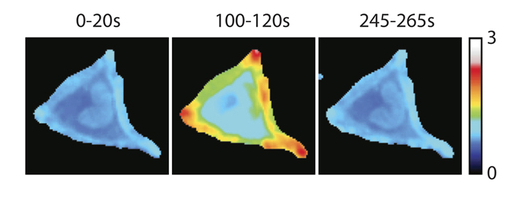
Research highlight: Subcellular location of signaling proteins determines cell shape
The small GTPase RhoA is best known for its ability to remodel the actin cytoskeleton of cells. To study the mechanism underlying the spatiotemporal control of RhoA activity by GEFs, single cell imaging was performed with an improved FRET sensor reporting on the nucleotide loading state of RhoA. A synthetic system (based on rapamycin induced heterodimerization) was used to show that recruitment of RhoGEF activity to the plasma membrane, but not to the Golgi apparatus, is sufficient for RhoA activation and actin polymerization.
The research, carried out at Molecular Cytology (SILS-FNWI) and the van Leeuwenhoek Centre for Advanced Microscopy, was published in the journal Scientific Reports (www.nature.com/articles/srep14693).