Research
Molecular Cytology is the study of the dynamic architecture of living cells. Our central theme is ‘Self-organisation and signalling in living cells’. Self-organisation is the intrinsic property of matter to organise itself in a (dynamic) structure, whereas signalling implies the activity of gene-products to control a local activity, which can alter the local cellular architecture (e.g. driving morphogenesis). In order to achieve a certain 3D architecture in cells, these two important mechanisms work in concert. At Molecular Cytology both mechanisms are studied with emphasis on membrane-related architecture of living cells using advanced microscopy tools. The activities are connected to the Faculty of Science Spearhead programme on Systems Biology, where our contribution is on spatiotemporal systems biology of higher eukaryotes. The main research areas are:
Spatiotemporal organisation of cellular signalling and its relation tissue and organ development
(group leaders prof. dr. T.W.J. Gadella, dr. J. Goedhart & dr. M.A. Hink, dr M. Postm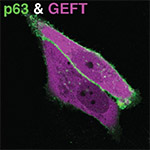 a, dr. R. van Amerongen, prof. dr. P.J. Hordijk)
a, dr. R. van Amerongen, prof. dr. P.J. Hordijk)
By employing genetic encoded fluorescent biosensors we analyse the in situ molecular interactions between signalling molecules (phospholipid-second messengers, receptors, G-proteins and effector molecules) & flow of information across and in the plane of the membrane of living mammalian cells. We aim to understand how cells can achieve and maintain a local signal in the membrane (e.g. in order to drive morphogenesis, or to define new cytoskeletal anchorage or vesicle-docking sites). The main pathways under study involve histamine/P2Y GPCR receptors, G-aq to PLC activation triggering downstream calcium, kinase signalling and small GTPase (Rho/Rac/Cdc24) signalling (the last in collaboration with prof. dr. P.J. Hordijk/Sanquin).
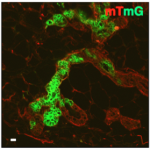 More recently we have started a new activity on developing models that include gene regulation and biomechanics of Nematostella vectensis embryogenesis. Hereby we use fluorescently labelled biomolecular markers to follow embryogenesis like adhesion molecules, cytoskeletal components (F-actin and Myosin) that are directly involved in cell shape changes and cell-cell interactions and also quantification of expression patterns. In November 2013 we were joined by dr. R. van Amerongen, a MacGillavry fellow of the Faculty of Science. She will set up a novel, independent research line focusing on the role of Wnt signaling in mammary gland stem cells and breast cancer.
More recently we have started a new activity on developing models that include gene regulation and biomechanics of Nematostella vectensis embryogenesis. Hereby we use fluorescently labelled biomolecular markers to follow embryogenesis like adhesion molecules, cytoskeletal components (F-actin and Myosin) that are directly involved in cell shape changes and cell-cell interactions and also quantification of expression patterns. In November 2013 we were joined by dr. R. van Amerongen, a MacGillavry fellow of the Faculty of Science. She will set up a novel, independent research line focusing on the role of Wnt signaling in mammary gland stem cells and breast cancer.
The close intertwining of several signalling cascades, their spatial organisational both within and between cells and our quantitative microscopy approach both necessitates and permits the generation of quantitative predictive modelling, which effectively will integrate this research line with Systems Biology approaches.
Advanced microscopy organised within the van Leeuwenhoek Centre for Advanced Microscopy (LCAM-FNWI)
(prof. dr. T.W.J. Gadella, dr. M. Hink, dr. Goedhart, dr. M. Postma and prof.dr. K. Jalink (special chair))
The goal of LCAM-FNWI is to boost Life Sciences research by implementing & developing (optical) microscopy techniques (see LCAM-FNWI/Research).
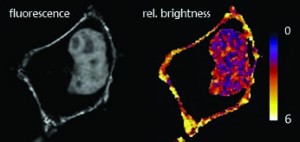 The main focus of LCAM-FNWI is centred around Functional Imaging Microscopy of living cells including multimode Fluorescence Resonance Energy Transfer (FRET), Fluorescence Spectral Imaging Microscopy (SPIM), Fluorescence Lifetime Imaging Microscopy (FLIM), Fluorescence Recovery After Photobleaching (FRAP) (dr. Gadella, dr. Goedhart & dr. Hink), & Fluorescence fluctuation microscopy, including Fluorescence (Cross) Correlation Spectroscopy (F(C)CS), Fluorescence Lifetime Correlation Spectroscopy (FLCS), Line-scan FCS, Number & Brightness techniques (dr. Hink), multicolor wide field and confocal (ratio) imaging (dr. Hink, dr. Goedhart, dr. Postma and dr. Gadella) including spectral and spinning disk confocal imaging.
The main focus of LCAM-FNWI is centred around Functional Imaging Microscopy of living cells including multimode Fluorescence Resonance Energy Transfer (FRET), Fluorescence Spectral Imaging Microscopy (SPIM), Fluorescence Lifetime Imaging Microscopy (FLIM), Fluorescence Recovery After Photobleaching (FRAP) (dr. Gadella, dr. Goedhart & dr. Hink), & Fluorescence fluctuation microscopy, including Fluorescence (Cross) Correlation Spectroscopy (F(C)CS), Fluorescence Lifetime Correlation Spectroscopy (FLCS), Line-scan FCS, Number & Brightness techniques (dr. Hink), multicolor wide field and confocal (ratio) imaging (dr. Hink, dr. Goedhart, dr. Postma and dr. Gadella) including spectral and spinning disk confocal imaging.
Other developments are at pushing new super-resolution microscopy techniques to the limit such as PhotoActivated Localization Microscopy (PALM) and Stochastic Optical Reconstruction Microscopy (STORM) and Super-resolution Optical Fluctuation Imaging (Gadella, Hink, Postma).
In each of the above approaches we invest strongly in quantitative data assessment (mostly driven by dr. Postma), since processing and evaluation algorithms are becoming more important, sophisticated and difficult. The extension of our research area towards multicellular 3D systems: cross endothelial cell migration (prof. Hordijk & coworkers) and mammary gland development (dr. van Amerongen) imposes new technical challenges, especially to enable quantitative in situ imaging of cell behavior and signal transduction in a complex three dimensional context.
 Of increasing importance for advanced microscopy in biology is the development of fluorescent probes with enhanced properties and –derived molecular biosensors. We have activities for enhancing intrinsic fluorescent protein brightness by mutagenesis & screening (dr. Gadella, dr Goedhart, dr Hink), for development of new photoswitchable fluorescent proteins (dr. Gadella and dr. Hink) and for new FRET-based biosensors reporting on second messenger levels, and on heterotrimeric & small G-protein activities in live cells (Goedhart & Gadella).
Of increasing importance for advanced microscopy in biology is the development of fluorescent probes with enhanced properties and –derived molecular biosensors. We have activities for enhancing intrinsic fluorescent protein brightness by mutagenesis & screening (dr. Gadella, dr Goedhart, dr Hink), for development of new photoswitchable fluorescent proteins (dr. Gadella and dr. Hink) and for new FRET-based biosensors reporting on second messenger levels, and on heterotrimeric & small G-protein activities in live cells (Goedhart & Gadella).